muscovite
What is mica?

Mica belongs to the phyllosilicate family, defined by its sheet-like (layered) atomic structure. The term "mica" encompasses over 30 mineral species, but only a few are commercially significant:
Muscovite: The most common mica species, composed of potassium aluminum silicate hydroxide (chemical formula: ). It is colorless to pale brown, translucent, and prized for its high dielectric strength and thermal stability (up to 700°C).
Phlogopite: A magnesium-rich mica with the formula . It exhibits golden-brown to copper hues and superior heat resistance (up to 1000°C), making it ideal for extreme-temperature applications.
Biotite: Iron-magnesium mica, darker in color (black to brown), but less industrially used due to lower insulation properties compared to muscovite and phlogopite.
All mica minerals share a hexagonal crystal system, allowing them to be cleaved into thin, elastic sheets—some as thin as 0.001 mm—without losing mechanical strength.
2. Physical Properties of Mica
Mica’s utility stems from its remarkable physical characteristics, tailored by its crystalline structure:
Thermal Conductivity
Mica exhibits low thermal conductivity (0.2–0.3 W/m·K), meaning it resists heat transfer. This property makes it a critical thermal insulator in high-temperature equipment like industrial furnaces, automotive engines, and electronic devices. For example, phlogopite mica is used in kilns and foundries to line heating elements, protecting surrounding components from excessive heat.
Electrical Conductivity & Insulation
One of mica’s most valued traits is its excellent electrical insulation. It has a dielectric strength exceeding 20 kV/mm and volume resistivity greater than , making it ideal for insulating high-voltage electrical systems. Muscovite mica, in particular, is used in capacitors, transformers, and cable insulation, where it prevents electrical arcing and short circuits.
Mechanical Properties
Flexibility & Toughness: Mica sheets are highly flexible and can be bent without breaking, allowing them to conform to complex shapes in industrial components.
Hardness: Mica has a Mohs hardness of 2.5–3, making it soft enough to be cut with a knife but durable enough to withstand mechanical stress in applications like gaskets and washers.
Cleavage: Mica’s perfect basal cleavage enables the production of ultra-thin sheets, critical for precision applications in electronics and optics.
| Physical Property | Description |
| Color | Diverse, varying by type: Muscovite is mostly colorless, transparent or light-colored (pale yellow, light gray); Biotite is dark brown, blackish brown or black; Phlogopite is golden yellow, brownish yellow or reddish brown, etc. |
| Luster | Vitreous luster; cleavage surfaces often exhibit pearly luster. |
| Hardness (Mohs) | 2~3, relatively soft; can be slightly scratched with a fingernail. |
| Cleavage | Perfect cleavage, with one set of cleavage parallel to the base; easily split into thin flakes, and the thickness of flakes can be as thin as micrometer scale. |
| Specific Gravity | 2.7~3.0, with slight differences among types (e.g., muscovite is about 2.76; phlogopite is about 2.7~2.85). |
| Transparency | Most are translucent to transparent; muscovite has high transparency, and its thin flakes can transmit light; biotite has lower transparency. |
| Elasticity | Thin flakes have significant elasticity; peeled flakes can be bent (with a small bending radius) and can return to their original shape after the external force is removed. |
| Insulating Property | Excellent electrical insulator, especially muscovite, with high insulation resistance and high dielectric strength. |
| Heat Resistance | Good high-temperature resistance: Muscovite can withstand temperatures of about 700°C; phlogopite has higher temperature resistance (800~1000°C); biotite has slightly lower temperature resistance (about 500°C). |
| Chemical Stability | Relatively stable chemical properties; resistant to acids and alkalis (except hydrofluoric acid); strong weathering resistance (muscovite is better than biotite). |
3. Chemical Properties
Mica is chemically inert and resistant to most acids, alkalis, and organic solvents, enhancing its durability in harsh environments:
Chemical Stability: It does not react with water, oxygen, or common industrial chemicals, ensuring long-term performance in outdoor or corrosive settings.
Thermal Decomposition: While stable at high temperatures, mica releases water vapor above 600°C (muscovite) or 800°C (phlogopite), a property carefully managed in high-heat applications like aerospace components.
| Chemical Property | Description |
| Chemical Classification | Belongs to layered silicate minerals, with a chemical structure based on silicate tetrahedron layers, containing interlayer metal cations (e.g., K⁺, Na⁺, etc.). |
| Main Chemical Composition | Varies by type: - Muscovite: KAl₂(AlSi₃O₁₀)(OH)₂ (contains potassium and aluminum) - Biotite: K(Mg,Fe)₃(AlSi₃O₁₀)(OH)₂ (contains potassium, magnesium, and iron) - Phlogopite: KMg₃(AlSi₃O₁₀)(F,OH)₂ (contains potassium and magnesium; fluorine can replace part of hydroxyl groups) |
| Acid and Alkali Resistance | Has strong resistance to common acids (e.g., hydrochloric acid, sulfuric acid, nitric acid) and alkalis (e.g., sodium hydroxide) at room temperature, not easily corroded; however, it is easily decomposed by hydrofluoric acid (HF) due to the reaction between the silicate structure and fluoride ions. |
| Chemical Stability | Chemically stable at room temperature, not easily reacting with air, water, or common chemicals; has strong weathering resistance, with muscovite being more stable than biotite (biotite is prone to oxidative weathering due to its iron content). |
| Thermochemical Stability | Stability at high temperatures varies by type: - Muscovite: Starts to dehydrate and decompose above ~700°C, leading to structural damage - Phlogopite: Higher temperature resistance (800~1000°C), more stable at high temperatures due to fluorine replacing hydroxyl groups - Biotite: Prone to structural changes due to iron oxidation above ~500°C. |
| Solubility | Insoluble in water and most organic solvents; only slowly dissolves under strong corrosive conditions (e.g., long-term exposure to concentrated hydrofluoric acid). |
| Ion Exchangeability | Interlayer cations (e.g., K⁺) can undergo slight ion exchange under specific conditions, but the exchange capacity is weak, and the chemical inertness is strong. |
4. Industrial Applications
Mica’s unique blend of properties makes it indispensable across industries:
Electronics & Electrical Engineering
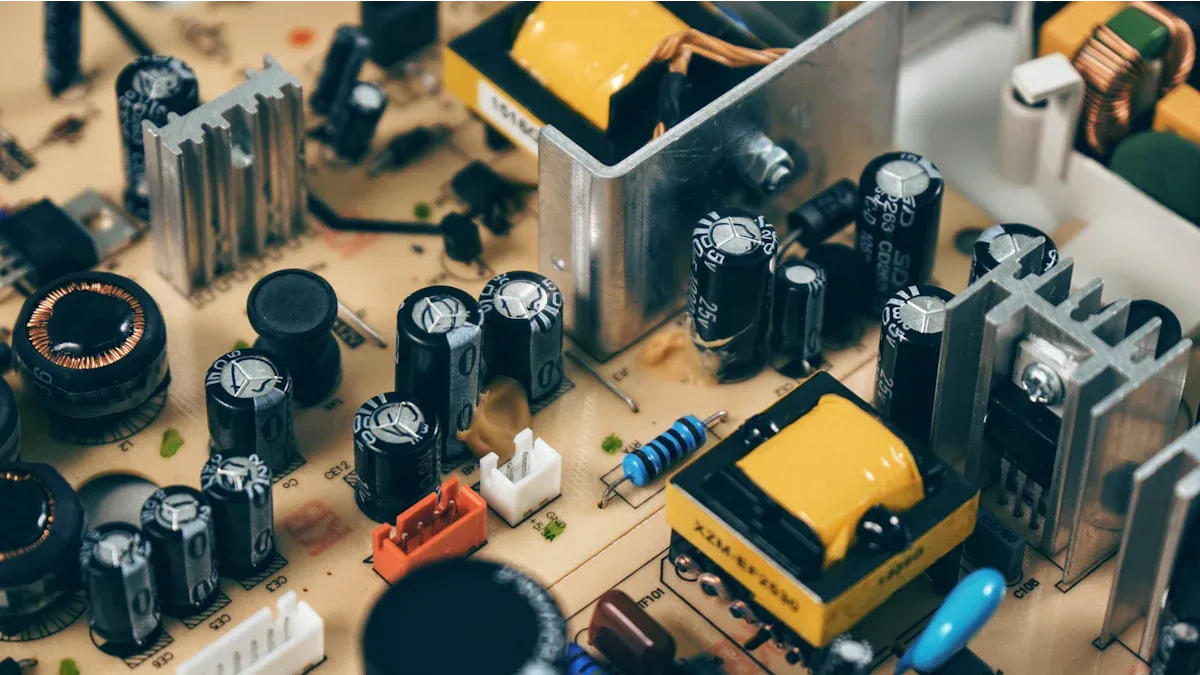
Insulation: Mica sheets insulate windings in electric motors, generators, and transformers, ensuring efficient energy transfer without electrical leakage.
Circuitry: Thin mica films are used as substrates in flexible electronics and high-frequency circuits due to their low dielectric loss.
Energy & Automotive

Battery Systems: Mica-based composites (e.g., mica tape, mica tubes) are critical in lithium-ion batteries for electric vehicles (EVs), providing thermal runaway protection. For instance, Yuefeng Mica’s mica tubes are used in EV battery packs to insulate high-voltage wiring, preventing heat spread during thermal events.
Renewable Energy: Mica insulates components in solar inverters and wind turbine generators, withstanding temperature fluctuations and humidity.
Manufacturing & High-Temperature Equipment
Industrial Furnaces: Mica bricks and boards line furnace walls, crucibles, and molten metal handling equipment, leveraging their heat resistance and low thermal conductivity.
Aerospace: Mica is used in rocket nozzles and satellite components, where it insulates against extreme temperatures and radiation.
Consumer Products
Cosmetics: Mica flakes add pearlescent effects to makeup (e.g., eyeshadows, lipsticks) due to their light-reflective properties.
Appliances: Mica heating elements in toasters, hair dryers, and electric stoves ensure uniform heat distribution while insulating users from electrical hazards.
5. Conclusion: Mica as a Foundation of Modern Technology
From ancient civilizations using mica for window panes to cutting-edge EV battery systems, mica remains a cornerstone of material science. Its unparalleled combination of thermal insulation, electrical resistivity, flexibility, and chemical stability makes it irreplaceable in industries demanding reliability under extreme conditions. As technology advances—particularly in renewable energy, electronics, and aerospace—mica’s role will only grow, solidifying its status as a "mineral of the future."
Previous Page
Next Page











